Energy Content of Fuels
In this experiment, you will find and compare the heat of combustion of two different fuels: paraffin wax and ethanol. Paraffin is a member of a group of compounds called alkanes that are composed entirely of carbon and hydrogen atoms. Many alkanes, such as gasoline and diesel oil, are important fuels. Ethanol, C2H5OH, is used as a gasoline additive (gasohol) and as a gasoline substitute. In this experiment, you will compare the energy content of paraffin and ethanol by measuring their heats of combustion in kJ/g of fuel.
In order to find the heat of combustion, you will first use the energy from burning ethanol or paraffin to heat a known quantity of water. By monitoring the temperature of the water, you can find the amount of heat transferred to it, using the formula
q = Cp•m•∆t
where q is heat, Cp is the specific heat capacity of water, m is the mass of water, and ∆t is the change in temperature of the water. Finally, the amount of fuel burned will be taken into account by calculating the heat per gram of fuel consumed in the combustion.
OBJECTIVES
In this experiment, you will
• Compare the heat of combustion for paraffin wax and ethanol.
• Calculate the heat of combustion and percent efficiency for both fuels.
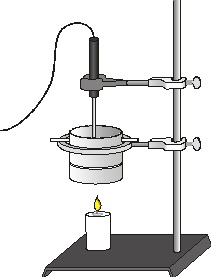
Figure 1
MATERIALS
LabQuest |
candle |
LabQuest App |
2 stirring rods |
Temperature Probe |
7.5 X 12.5 cm (3" X 5") index card |
100 mL graduated cylinder |
small can |
ring stand and 10 cm (4 inch) ring |
cold water |
utility clamp |
matches |
alcohol burner with ethanol |
|
PROCEDURE
1. Obtain and wear goggles.
2. Connect the Temperature Probe to LabQuest and choose New from the File menu. If you have an older sensor that does not auto-ID, manually set up the sensor.
3. On the Meter screen, tap Rate. Change the data-collection rate to 0.5 sample/second (interval of 2 seconds/sample) and the data-collection length to 600 seconds. Data collection will last 10 minutes. Select OK.
Part 1 Paraffin Wax
4. Prepare a candle for the experiment by holding a lighted match near its base, so that some melted wax falls onto a 7.5 X 12.5 cm (3" X 5") index card. Immediately push the candle into the melted wax and hold it there for a moment to fasten it to the card.
5. Find and record the combined mass of the candle and index card (or the alcohol burner and contents in Part 2 below).
6. Determine and record the mass of an empty can. Add 100 mL of chilled water to the can (use 200 mL of chilled water in Part 2). Obtain the chilled water from your teacher. Determine and record the mass of the can and water.
7. Set up the apparatus as shown in Figure 1. Use a ring and stirring rod to suspend the can about 5 cm above the wick. Use a utility clamp to suspend the Temperature Probe in the water. The probe should not touch the bottom of the can. Remember: The Temperature Probe must be in the water for at least 30 seconds before you do Step 8.
8. Start data collection. Monitor temperature (in °C) for about 30 seconds and record the initial temperature of the water, t1, in your data table. Then light the candle (or alcohol burner in Part 2). Heat the water until its temperature reaches 40°C and then extinguish the flame. CAUTION: Keep hair and clothing away from an open flame.
9. Continue stirring the water until the temperature stops rising. Record this maximum temperature, t2. Data collection will stop after 10 minutes (or stop before 10 minutes).
10. Determine and record the final mass of the cooled candle and index card, including all drippings (or the cooled alcohol burner and contents in Part 2).
11. To confirm the initial (t1) and final (t2) values you recorded earlier, examine the data points along the curve on the displayed graph. As you tap each data point, the temperature and time values are displayed to the right of the graph.
Part 2 Ethanol
12. Repeat Steps 5–11 using ethanol in an alcohol burner. Be sure to use 200 mL of chilled water in Step 6.
Processing the data
1. Find the mass of water heated.
2. Find the change in temperature of the water, ∆t.
3. Calculate the heat absorbed by the water, q, using the formula in the introduction of this experiment. For water, Cp is 4.18 J/g°C. Change your final answer to kJ.
4. Find the mass of paraffin or ethanol burned.
5. Calculate the heat of combustion for paraffin and ethanol, in kJ/g. Use your Step 3 and Step 4 answers.
6. Calculate the % efficiency in both trials of the experiment. Divide your experimental value (in kJ/g) by the accepted value, and multiply the answer by 100. The accepted heat of combustion of paraffin is 41.5 kJ/g, and for ethanol the value is 30.0 kJ/g.
7. Based on your results, which fuel produces more energy per gram burned? Give an explanation for the difference. (Hint: Ethanol, C2H5OH, is an oxygenated molecule; paraffin, C25H52, does not contain oxygen.)
8. Suggest some advantages of using ethanol (or paraffin) as a fuel.
9. Discuss heat loss factors that contribute to the inefficiency of the experiment.
DATA and calculations
|
Part 1: Paraffin |
Part 2: Ethanol |
Initial mass of fuel + container |
g |
g |
Final mass of fuel + container |
g |
g |
Mass of fuel burned |
g |
g |
Mass of can and water |
g |
g |
Mass of empty can |
g |
g |
Mass of water heated |
g |
g |
Final temperature, t2 |
°C |
°C |
Initial temperature, t1 |
°C |
°C |
Temperature change, ∆t |
°C |
°C |
Heat, q |
kJ |
kJ |
Heat of combustion, in kJ/g |
kJ/g paraffin |
kJ/g ethanol |
% efficiency |
% |
% |